Photosynthetic Hydrogen Production
The forced delivery of PSI generated electrons to an artificial ferredoxin-hydrogenase fusion enzyme can be used for the mass production of hydrogen gas. This, together with the creation of an engineered PSI for accepting electrons from multiple donors, should make the photosynthetic production of hydrogen a feasible reality.
The forced delivery of PSI generated electrons to an artificial ferredoxin-hydrogenase fusion enzyme can be used for the mass production of hydrogen gas. This, together with the creation of an engineered PSI for accepting electrons from multiple donors, should make the photosynthetic production of hydrogen a feasible reality.
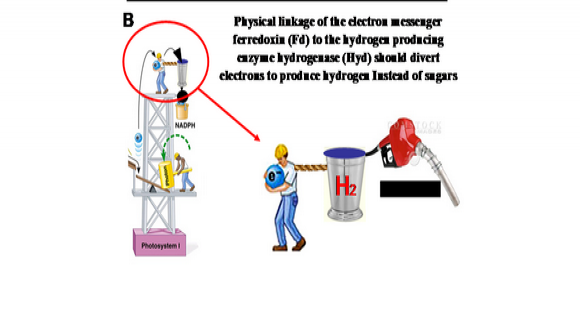
Oxygenic photosynthesis underpins the survival of virtually all higher forms of life. Photosystem I (PSI) generates the most negative redox potential in nature. Most of the negative redox potential is stabilized in the form of reduced ferredoxin (Fd) that serves as an electron donor to the enzyme ferredoxin-NADP+-reductase (FNR). Under normal physiological conditions, Fd reduces NADP+ to NADPH via the Fd-FNR complex. NADPH is the main reducing force in the Calvin cycle, in which organic carbon in the form of CO2 gas is fixed into glucose, the main source for organic carbon in nature. In some systems there is competition between FNR and the hydrogenase for the PSI generated electrons. Hydrogenases are present in only part of the photosynthetic world, namely unicellular algae and cyanobacteria, and are expressed only under anaerobic condition.
To be able to shuttle most of the PSI generated electrons to the hydrogenase, the competing processes must first be eliminated or bypassed. In our research we will be interfering with the formation of the naturally occurring Fd-FNR complex by over-expressing artificial ferredoxin-hydrogenase fusion enzymes. Our aim is to force the delivery of PSI generated electrons to the artificial ferredoxin-hydrogenase fusion enzyme and in this way be used for mass hydrogen gas production. An additional aspect of the study, carried out in Prof. Nelson’s Laboratory, is the creation of an engineered PSI which, as a result of subunit fusions inspired by PSI from marine phages, should be promiscuous in accepting electrons from multiple donors, thereby increasing its throughput. The two technologies combined should make photosynthetic hydrogen production a feasible reality.
collaboration Dr. Iftach Yacoby of The Center for Biomedical Engineering (MIT)

Figure 1: Photosynthetic hydrogen production. During the natural course of photosynthesis (A), electrons from Photosystem I (PSI) are shuttled by ferredoxin (Fd) mostly to NADP+ reductase (large red arrow) and utilized for energy storage by the plant or alga. In our approach (B) fusion of Fd to hydrogenase should shuttle more electrons directly towards H2 production.